Background and Significance: Natural killer (NK) cells are cytotoxic lymphocytes that play a key role in recognizing malignant cells. In AML, qualitative and quantitative dysfunction of innate NK cells portends a poor prognosis and adoptive NK cell therapy has been shown to be safe and effective with minimal risk for GVHD (Ciurea et al. 2022; Silla et al. 2021). To circumvent cost and logistics restraints of per patient manufacture of NK cells, we developed a universal donor (UD) NK cell bank for “off-the-shelf” NK cells (UD FC21-NK cells). Donors with optimal NK cell characteristics are identified through the National Marrow Donor Program, and peripheral blood NK cells are expanded for two weeks by co-culture with irradiated K562 feeder cells expressing 4-1BB ligand and membrane bound IL-21 as previously described (Denman et al. 2012). We seek to study the safety and efficacy of multiple doses of UD FC21-NK cells combined with fludarabine and cytarabine (FLA) chemotherapy in patients with relapsed or refractory AML.
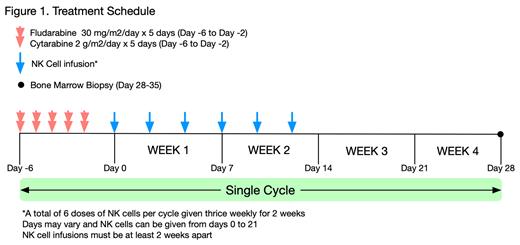
Study Design and Methods: This open label, single-center phase I/II study will include a dose escalation phase to establish safety and determine the recommended phase II dose followed by an expansion phase to estimate efficacy (complete response rate and MRD negative response rate). Up to 20 patients ages 18-25 with relapsed or refractory AML, including isolated extramedullary disease, will be enrolled. Exclusion criteria include active GVHD, uncontrolled infections, hematopoietic stem cell transplant within 3 months of enrollment, and donor lymphocyte infusion within 30 days of enrollment. Patients will undergo FLA chemotherapy followed by six doses of UD FC21-NK cells given thrice weekly for two weeks (Figure 1). Dose levels include 1 x 10 7 UD FC21-NK cells/kg/dose, 3 x 10 7 UD FC21-NK cells/kg/dose, and 1 x 10 8 UD FC21-NK cells/kg/dose. Patients may receive up to 2 cycles if they do not achieve a CR after cycle 1 or if necessary to bridge to transplant. Peripheral blood samples will be drawn weekly for immune correlatives including in vivo NK cell expansion and persistence. The trial is currently open to enrollment to adult patients with the intent to expand to pediatrics after demonstrating preliminary safety. Clinical trial information: NCT05503134
Disclosures
Lamb:Kiadis Pharma, a Sanofi company: Research Funding. Lee:Kiadis Pharma, a Sanofi Corporation: Consultancy, Patents & Royalties: licensed through Nationwide Children's Hospital; Avidicure B.V.: Consultancy, Current equity holder in private company, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal